November 1, 2024
XBiotech announces the completion of non-clinical data and is preparing to submit Investigational New Drug (IND) application for their new Unmetix™ cocktail. Unmetix is a novel therapeutic being developed by the company to treat patients with Shingles and the associated post-herpetic neuralgia (PHN). Shingles is caused by Varicella Zoster Virus (VZV). VZV is a human herpesvirus responsible for chickenpox (varicella), shingles (herpes zoster), and postherpetic neuralgia.
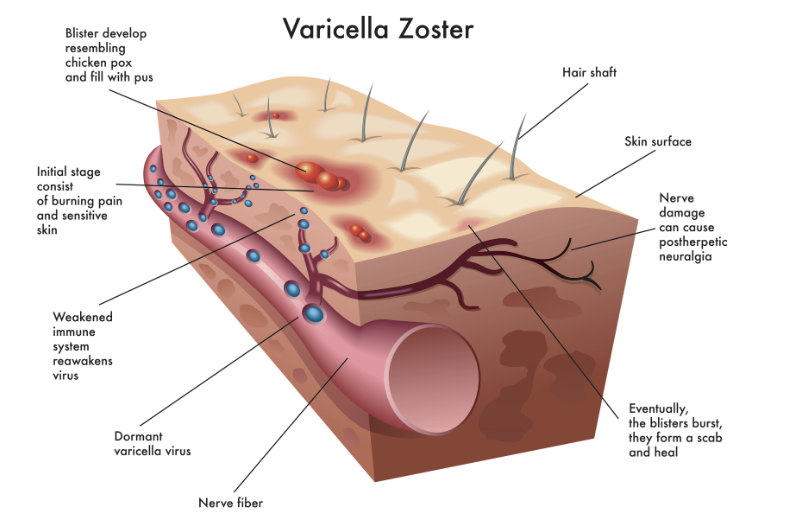
The primary infection by VZV occurs mostly in children, and initiates in the respiratory mucosa, followed by dissemination of the virus particles to the skin resulting in itchy rash with small fluid filled blisters. Following an acute phase of chickenpox with VZV, the virus stays dormant in the nerve cells, and reactivation from latency later in life leads to the manifestation of herpes zoster. Herpes zoster or shingles, is the clinical manifestation of the VZV reactivation, mainly seen in older adults or immunocompromised humans. Shingles can occur anywhere in the body, and manifests as a single stripe of painful blisters on one side of the torso. A common complication of shingles is long term nerve pain called postherpetic neuralgia (PHN). About 10-20% of patients with shingles experience PHN. About 1 in 3 people in the United States will develop shingles in their lifetime, with risk increasing with age and weakened immune system. Antivirals currently being used to treat shingles have limited effected on the virus, and no effect on PHN.
Unmetix is a combination of two monoclonal antibodies: XB22-14, which is a neutralizing anti-herpes zoster antibody, and XB2001 (vilamakitug), which targets interleukin-1𝜶 (IL-1𝜶). Both antibodies are True Human™, derived without modification from naturally occurring human immunoglobulin sequence. Unmetix is intended to not only neutralize the virus, but also reduce pain and discomfort cause by PHN. In vitro and in vivo studies with Unmetix shows high potency against VZV infection caused by various genotypes of the virus, ideal pharmacokinetics, and enhanced safety. The company plans to file the new drug application with the FDA in Q1 of 2025, and start clinical testing subject to FDA approval.