May 17, 2023
The study uses sequential dose escalation involving three groups (or cohorts) of subjects to prove Hutrukin tolerability. Participants receive a single intravenous infusion of either placebo or active drug Hutrukin at either 1,000 mg (Cohort 1), 3,000 mg (Cohort 2), or 5,000 mg (Cohort 3) dose levels. Dose escalation will utilize a 6+2 design with sentinel dosing and doses will be explored with 8 subjects in each cohort (6 subjects are dosed with Hutrukin and 2 subjects with placebo).
Subjects in Cohort 1 have now completed dosing and safety evaluation. Hutrukin was well tolerated in all subjects. The study will now begin enrolling subjects into Cohort 2.
Hutrukin is a novel treatment to reduce brain injury as part of urgent therapy for stroke victims. If a blood clot occurs in an artery that supplies blood to the brain, the loss of blood supply starves a portion of the brain of oxygen (ischemia). This is known as an ischemic stroke. Clot-busting drugs and/or catheters can be used to re-open the clog artery. However, surprisingly, after opening of the clogged artery, return of blood supply to ischemic brain can result in aggravation of ischemic injury, often with a rapid expansion of brain damage.
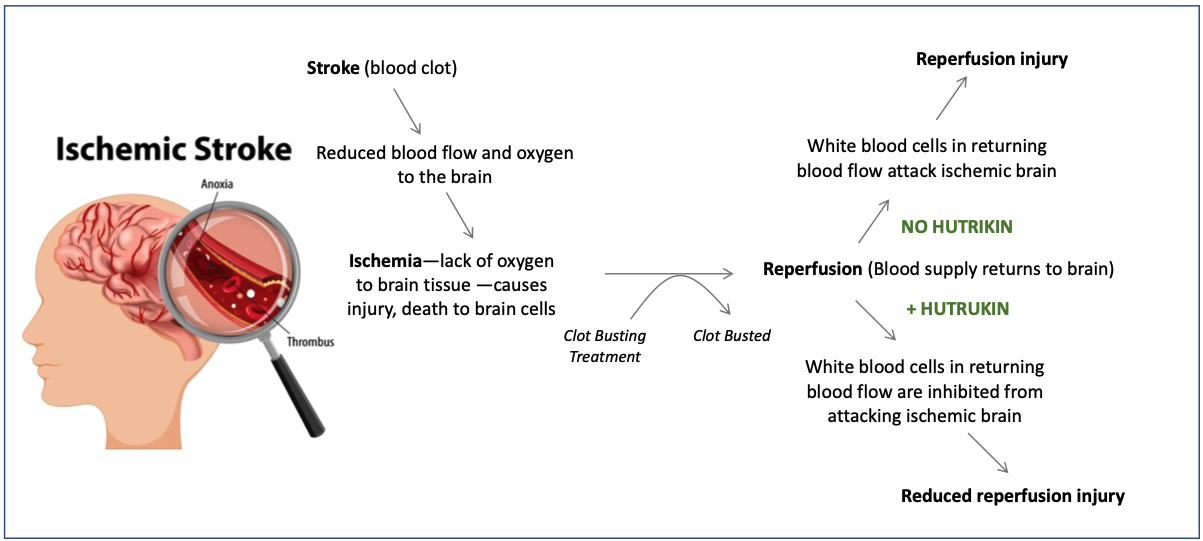
Hutrukin is intended to be given immediately prior to clot-busting procedures. Hutrukin is intended to reduce reperfusion injury associated with the resumption of blood supply to the hypoxic brain tissue. There is currently no drug available to treat reperfusion injury. Hutrukin, therefore, has the potential to be a novel, breakthrough therapy in the management of ischemic stroke. Stroke is the second most common cause of death or disability worldwide.